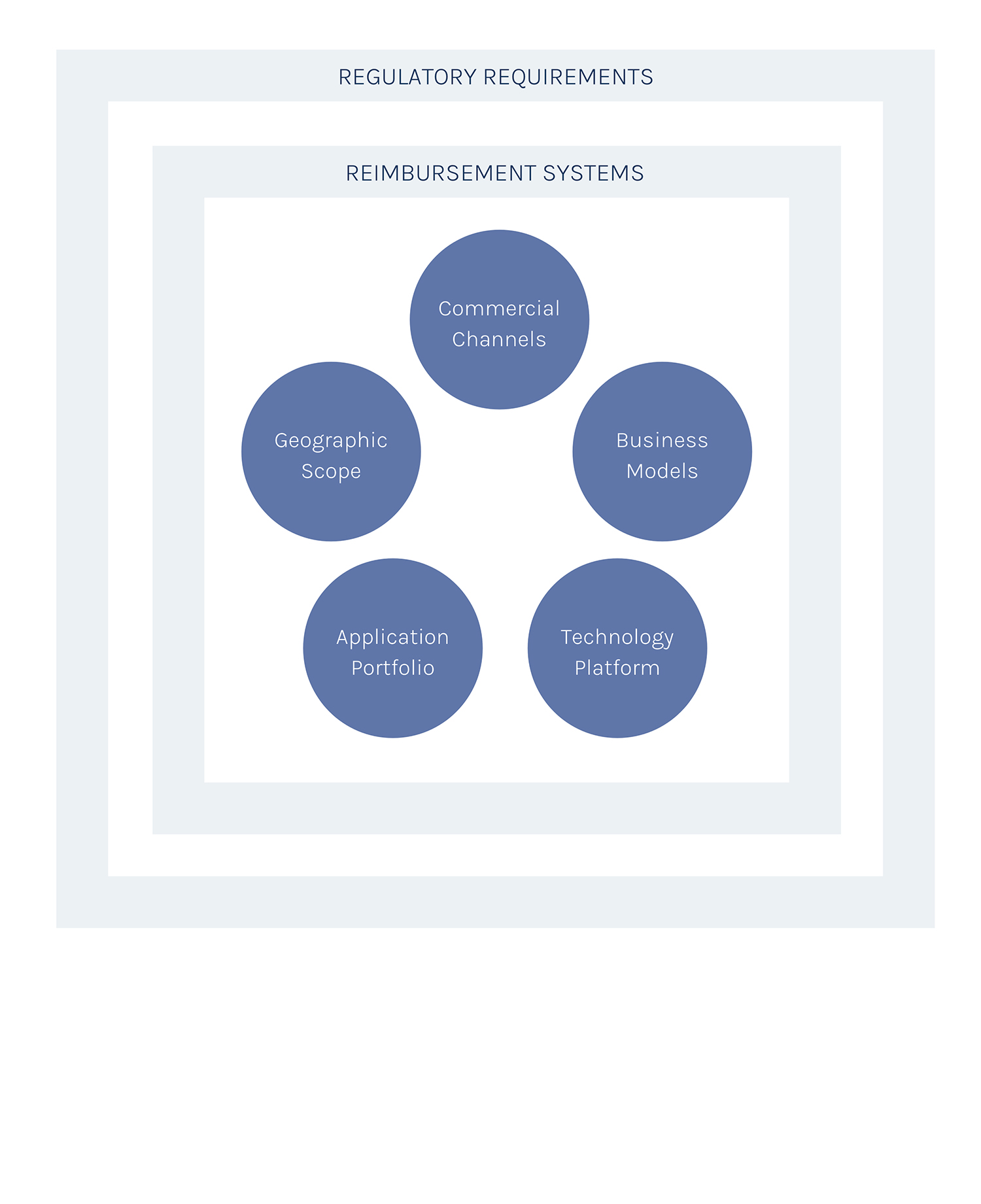
Long-term strategy and growth model
Introducing a new concept or technology is tough in any market. It’s especially complex in the IVD/healthcare space with its rules, regulations and guidelines that safeguard patients’ health and safety. Moreover, we operate within the extremely demanding IVD-D/IVD-R Class C regulatory environment. And our product/service is subject to different reimbursement systems in each European market. To address this significant complexity, we have a 5-pronged approach.
Commercial Channels
We have agreements in place with established IVD distributors to ensure fast rollout with minimum upfront investment from our side.
Business models
We started with our Testing Service based in our centralized lab, owned and run by 2cureX. We will transition to hospital in-house testing when the technology is ready.
Technology Platform
Focus on quality of the patient’s tumor replicas, turnaround time, adaptation to new drug mechanisms and convenience (including developing a system that can be used for in-house testing at hospitals).
Application portfolio
Develop specific tests for each therapy decision-making point, starting at the end (mCRC 3rd line) and moving upstream to cover the whole patient pathway.
Geographic scope
Start in Europe, in as many countries in parallel as possible, to address the different reimbursement schemes. Expand to other Regions when the hospital in-house testing system is ready.